The world around us is changing rapidly, from machine learning to cryptocurrencies, every new breakthrough claims to be the ‘thing’ that replaces the old, that changes the game, that revolutionizes the economy. Yet, there are only a few things that have made slow but honest progress in the past years, things that really do cause change. For example, the advancements in computing power have been quite predictable for the past decade, but the consequences of this steady growth are widespread. From being able to handle the large amounts of math required for machine learning to dealing with the computations involved with the Blockchain. These slow-growth technologies are the areas to look out for, as they power advancement in many fields besides their own. One such technology that has slowly been changing hundreds of industries from cars to medicine to warfare is the battery:
Despite what some tech blogs may claim, there has been no revolutionary breakthrough in battery technology in the past couple years, there has only been slow and steady progress, and the reason behind this lack of breakthroughs is fascinating. Yet, in order to understand why human ingenuity has not rushed progress with batteries, we must first understand the basics:
When you look at any material, you find that there are properties associated with it: metals are malleable, gems are hard, water is wet. These properties all arise out of what the material is made out of at the most basic level: protons, neutrons, and electrons. Now it is no simple task being able to guess the properties of something based off of what’s inside, but it is important to note that the composition determines how a material acts with other materials. Since all things are made out of the same things, all things interact. Simply by touching something else you are interacting with it at the most fundamental level. The electrons in your hands are mingling with everything around them, often times they simply leave your hand and join the things they touch.
The reason behind this is because all things like to be lazy. It is a universal truth, if there is a possibility for two materials to both get to a lazier state, then they will do so (at least on average). And often times in order for two materials to both get to a lazier state they have to trade with each other. One material will give another some electrons while the other material will give some ‘ions’ (which are just smaller bits of materials that are also made up of protons, neutrons, and electrons). This trade is what we call an interaction. The reason that we care about these interactions is because occasionally we can tap into the stream of electrons going from one material to another and get something amazing out: electricity. So now you see why we may be incentivized to look a little closer and understand what’s really going on. If we can make the interactions big enough, then we can harness the energy that comes with them to power our modern lives.
Yet, this is where the first problem with batteries arise. If we allow a reaction to get too crazy, there is no way for us to harness all the energy at the same time, instead of gathering up all that energy we simply end up making a bomb. But, at the same time we also need a substantial interaction in order to get some energy that makes the effort worth our time. So when it comes to chemical interactions, it is a balancing act between bombs and trickles.
This first problem was solved with some smart engineering and led us to the structure of a battery that we still use today. There are three parts: the anode, cathode, and electrolyte. Simply put, the anode and cathode are two materials that really like to interact with one another, and the electrolyte is a special material that usually prevents them from doing so, but when prompted which actually catalyze the reaction, making it faster and more efficient. It is the control valve of the battery, allowing us to make an interaction worth our while, while also preventing us from making a bomb (but if we break the battery open and reduce the effectiveness of the electrolyte, an explosion is still possible, which is why we can’t bring all batteries onto airplanes).
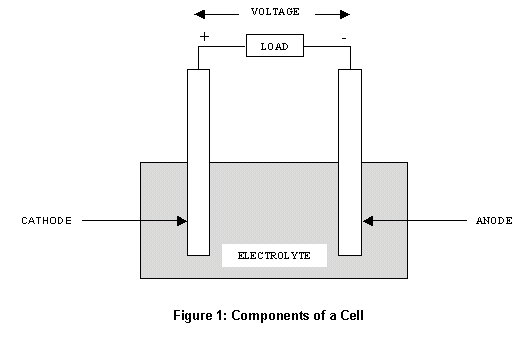
Now when we connect the anode to the cathode bypassing the electrolyte, we are essentially giving the two materials free rein to interact, so they start trading electrons and ions. The electrons which are initially sent at very high speeds and energies are siphoned off to power our lights and appliances and then returned very slow and tired. The ‘ions’ pass through the electrolyte and keep the reaction going for as long as we can make it. Eventually both materials will get to a lazier state and will no longer have a need to trade electrons and ions, leaving us with no more electricity and a ‘dead’ battery. This is the second problem that batteries bring: attempting to find the best combination of cheap materials that will result in the longest and strongest flow of trade possible.
Yet, as we see today, nobody wants single-use batteries. We want to be able to use a battery again and again, which complicates things. Some very smart scientists found out that if you push energy into the battery in a special way you can actually get the two materials to start trading again in the exact opposite manner, thus ‘undoing’ the reaction leaving the materials at high-energy state ready to trade once again. Thus, in this manner we can recharge some batteries, yet we are not reversing time, and we are not entirely undoing the reaction. In reality, some of the ions interact with the electrolyte in unexpected ways, as all things interact with another, and occasionally an ion will find itself very comfortable in a vey lazy state that we are not able to knock it out of. Thus, with every recharge cycle the battery is degraded as more ions find themselves too comfortable to be knocked back. And this is the third problem of batteries: reducing the degradation of batteries.
As you may be able to guess, these last two problems of finding good combinations of materials and reducing degradation are where nearly all of current battery research is centered. They require a deep understanding of materials science, chemistry and physics at the quantum level to even begin to think about, and even when you can understand the intricacies of the problems, you are no close to solving them. In fact, you may never fully understand the problems because there are so many factors involved with them that it is not even clear where to begin looking.
We can actually dive a little deeper, pretend that we are researchers looking at these problems. Up until this point, we have been thinking about protons, neutrons, and electrons as little objects that form everything and determine how things interact. Yet, when approaching these problems, that picture is inadequate. In fact, it will simply lead you to the wrong conclusions, especially in the computations of the physics behind it all. When trying to look at these problems, researchers have to use wave functions, which are just mathematical shortcuts which lead us to the probabilities of finding an individual particle, such as an electron, somewhere. Yet when you start looking at the larger picture and having dozens or hundreds of electrons and protons all interacting with one another all with their own wave functions, the math gets complicated. Actually, that is an understatement, the math gets nearly impossible. It is like trying to look at the ocean and make sense of the choppy water. It may seem like there is a pattern, but there is no way to know perfectly how every wave in the ocean was formed. There is simply too much going on: at the beaches people are splashing, there are hurricanes, tsunamis and underwater earthquakes, boats are crossing all over the place, and all the fish in the sea are swimming around causing their own disturbances
Earlier on in the process of research, many scientists tried to use their intuition in order to skip (or lessen) the math. They would test out different materials and see what kind of results they got from experimenting with them. Yet no significant progress has been made through intuition alone for more than thirty years, perhaps longer. Energy density has only improved five-fold in the past two centuries. In the past decade we have improved Lithium-ion batteries only marginally, but not due to any breakthroughs, rather primarily due to improvements in manufacturing and production. Tesla, and companies like it, are really pushing the limits on what we can do with batteries, but developments in research are not keeping up. Most manufacturers are only predicting a twofold increase in energy density in the next decade, meaning batteries will still be far behind fossil fuels for the foreseeable future.
We are still looking for better materials and more efficient methods, yet we are playing a slow guessing game. We plug in random materials into super computers hoping to find a combination whose interactions with one another may prove to be more effective than today’s standards. Many researchers are attempting to stick to Lithium and are attempting to pair it with other more exotic compounds (with some success), while others are experimenting with radically different materials such as graphene and sodium. Yet, there is no clear breakthrough in sight as research keeps trudging along. It is quite troubling to think that intuition and human ingenuity can only take us so far, but it is the sad truth. Things get too complicated for us to wrap out heads around at the atomic scale, so we have to rely on the absurdly complicated maths that we derived over the past century to take us the rest of the way.
In the meantime, companies have to make sacrifices. Tesla is using different types of battery for different purposes. In some of their older cars they used Nickel-Cobalt compounds for increased life-cycles, aka less degradation, while today they are using more classical Lithium-Ion pairs with better energy density, aka better materials. No one has created the perfect battery, thus innovation has filled the gaps where it can, like using ultra-capacitors to output energy at a much faster pace than batteries are capable. Yet despite the widespread claims on the internet of a new super-battery right around the corner, progress is slow, and the research is anything but glamorous. There are many leads to pursue, but we have no clear reason to be hopeful. There has not been a breakthrough in a very long time, so if the past is anything to rely on, we still have quite a lot of ground to cover before batteries can truly overcome the barriers set against them in the automotive and energy storage sectors, among others.
There are many sectors that have and can expect drastic and sudden improvements. Companies are just beginning to take advantage of the data that has been accumulated for the past two decades. The online market is still in its prime for reaching unknown and undiscovered markets. Computer Science is starting to see some amazing breakthroughs with machine learning. Many of these technologies are quick to become trendy catchphrases and words to drop in a conversation to make yourself self-sound intelligent. And many people have tried to shove batteries into that same group, but reality tells us that they are not ‘the next big thing’ any more than they were ten years ago. The only thing that has changed is our fascination with batteries and our willingness to experiment with what they can achieve. Like many of the fundamental technologies today, while things may seem to be moving too quickly to keep up, these technologies are slow to improve. The only things that are truly moving at a pace too rapid to keep up with are the uses of existing technologies that people can dream up and sell to one another. There is a reason we have thousands of the brightest minds working on basic fields like battery technology: the problems are not simple. And no matter how much we want something to revolutionize the way we live, it won’t unless we get innovative with what we already have.